Surround Medical wins FDA 510(k) for PORTRAY™
Katherine E. Pfaff
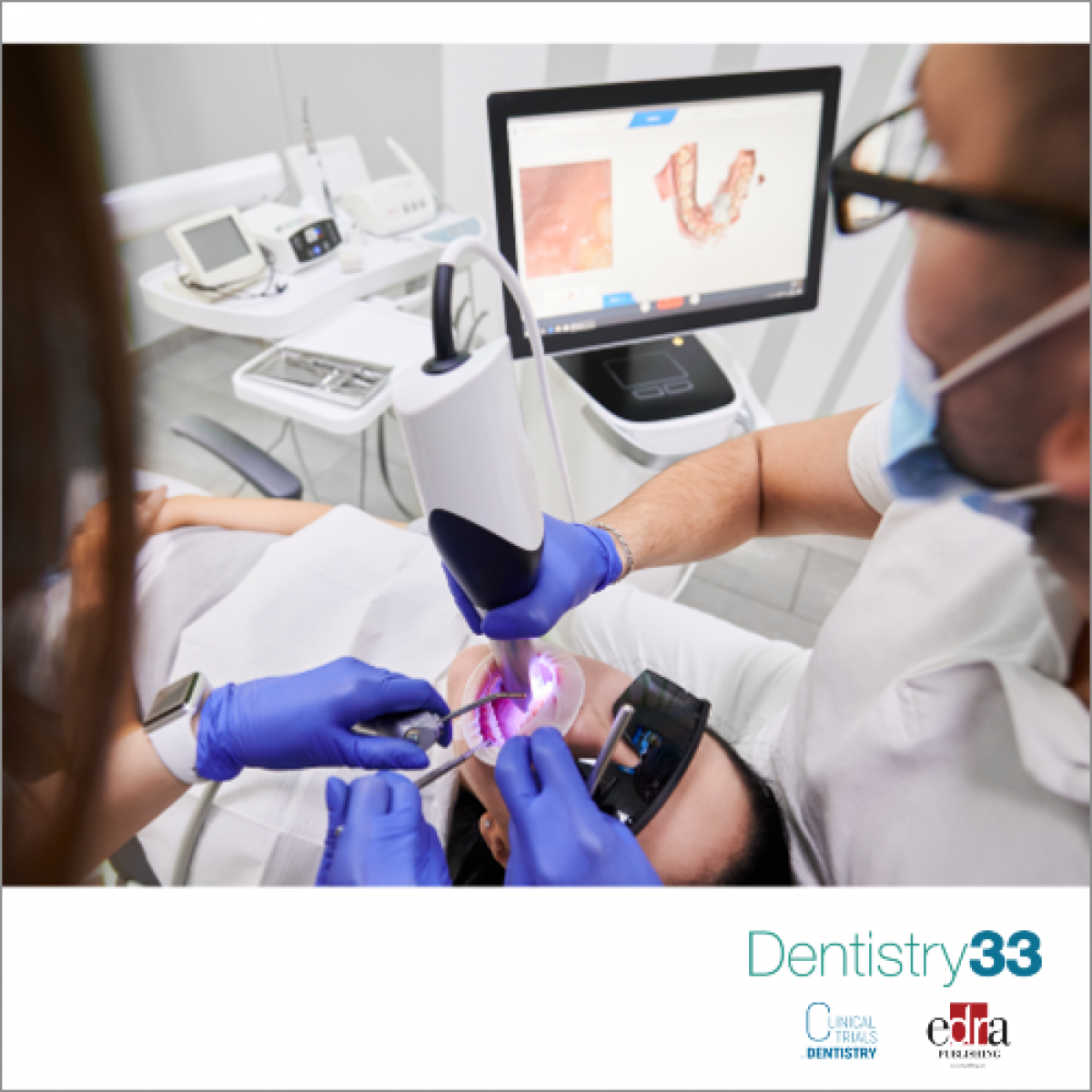
Surround Medical Systems, Inc. received FDA 510(k) pre-market clearance for the 2D diagnostic system, PORTRAY Dental Imaging System. The devices are expected to be available within the fourth quarter and will be prepared and shipped from the North Carolina facility.
Under the clearance, PORTRAY can be used as a 2D diagnostic tool, as well as to utilize additional diagnostic capabilities, including 2D synthetic and 3D tomosynthesis imaging to create radiographs of the jaw, teeth, and mouth. The advancement could improve cavity detection compared to x-rays by providing imaging of early tooth decay and improved detail to see additional dental issues.
"Dentists have been waiting for a major improvement to intraoral dental imaging for more than two decades," said David LaVance, president and CEO. "The PORTRAY system provides dentists with an intraoral 3D dental imaging system that will allow the dentists to provide a higher level of care for patients by detecting cavities and fractures that would otherwise go undetected by current 2D intraoral dental imaging systems. The PORTRAY system has the potential to substantially change and improve dental techniques and provide patients better outcomes and improved health."
PORTRAY was developed based on carbon nanotube technology that was built on x-ray methodology. The discoveries were made by the University of North Carolina at Chapel Hill researchers.
 Related articles
Related articles
Products 26 November 2024
DentalMonitoring Enhances Orthodontic Software With FDA-Cleared AI Technology
DentalMonitoring, the leader in artificial intelligence (AI)-powered remote monitoring for orthodontics, has released a major software update introducing features and indications based on the...
News 15 October 2024
Relu Secures FDA Clearance and CE Mark Approval for Revolutionary Dental AI Tool
Relu, a pioneer in artificial intelligence (AI)-assisted segmentation for dental labs and software companies, proudly announces the dual achievement of 510(k) clearance by the U.S. Food and Drug...
Market 11 April 2024
Orthobond Revolutionizes Medical Device Safety With FDA Approval of Ostaguard Coating
Orthobond Corporation, a leader in covalently-bound antibacterial surface technologies with broad applications in the medical device industry, announced that the U.S. Food and Drug Administration...
AIOMEGA, a Texas biomedical company, announced that AIO BREATHE, their medical device that treats Obstructive Sleep Apnea, has been cleared by the Food and Drug Administration.
The U.S. Food and Drug Administration (FDA) has approved Dr. Jonathan An from the UW Department of Oral Health Sciences to lead the first-ever study to evaluate rapamycin in older adults with...
 Read more
Read more
Oral surgery 27 October 2025
The authors assessed the incidence of postoperative bleeding in patients who were highly anticoagulated and in patients who underwent extensive oral surgical procedures and who continued using oral...
With a new name, Tufts Special Care Dental Clinics continues a 50-year mission of treating people with intellectual and developmental disabilities
Ultradent Products, Inc., a leading developer and manufacturer of high-tech dental materials, is announcing the launch of VALO X Colors: new, vibrant finishes for the award-winning VALO X curing...
News 27 October 2025
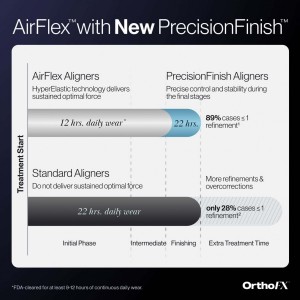
OrthoFX, a leading innovator of shorter wear time aligner systems, announces AirFlex with new PrecisionFinish Aligners.
News 27 October 2025
Young Innovations Strengthens North American Leadership Team
Young Innovations, a leading global manufacturer and distributor of dental supplies and equipment, is pleased to announce two significant additions to its North American leadership team as part of...