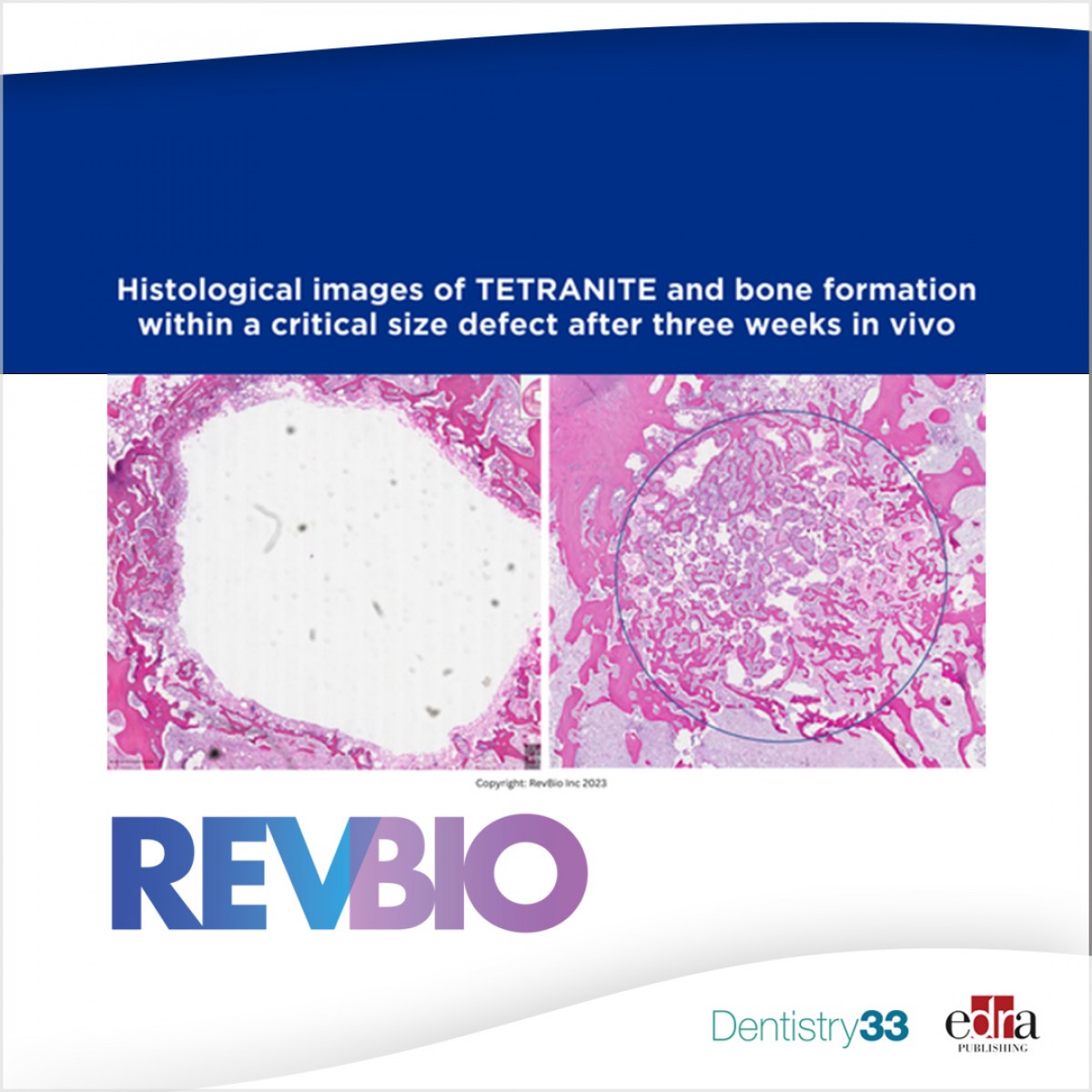
FDA okays RevBio dental implant stabilization clinical trial
RevBio, Inc., announced on Aug. 2 that it has received approval from the U.S. Food and Drug Administration to start a 20-patient clinical trial to examine the safety and efficacy of a pH modified porous formulation of bone adhesive biomaterial called Tetranite. This product can immediately stabilize dental implants following tooth extractions, according to a news release.
This new formulation has shown evidence of a more biologically active bone substitution. While not osteoinductive, this patent-pending version of Tetranite has shown characteristics which may be described as "osteopromotive."
The clinical trial will be conducted by Dr. Paul Fugazzotto, DDS, a world-renowned periodontist based in Massachusetts, who has over 30 years of experience placing dental implants, and Dr. Kanyon Keeney, DDS, an oral and maxillofacial surgeon based in Virginia whose clinical practice focuses exclusively on dental implant surgery.
"This product is truly transformational," said Fugazzotto in the news release. "Tetranite will revolutionize how implant dentistry will be performed. The adhesive properties and handling characteristics of this material are incomparable to any product on the market."
Teeth are extracted due to damage from traumatic injuries, tooth decay, or gum disease. The current standard of care consists of multiple staged surgical procedures to restore a patient’s dentition with prosthetic crowns supported by dental implants.
Frequently, extraction sites are too large for dental implants to achieve primary stability through conventional mechanical engagement. Instead, patients must undergo a costly, complex, and lengthy process including a preliminary bone grafting surgery before receiving a dental implant.
Based on company surveys, each year in the U.S. approximately 2.1 million implants are eventually placed into initially unstable tooth extraction sites that first must receive a bone graft. The use of Tetranite to stabilize an unstable implant will allow for the immediate placement of dental implants which otherwise could not be placed until the initial bone graft has healed to form new bone.
As a result, the Tetranite biomaterial will help reduce the duration and complexity of these dental implant procedures, lessen patient pain and recovery time, and reduce the overall cost of care thereby providing greater patient access for the treatment of tooth loss.
In the news release, Alan Pollack, RevBio's senior director of dental clinical operations, said that the improvements made to the technology have accelerated the bone substitution profile and the product's adhesive strength.
"Having also just recently received IRB approval, we look forward to enrolling our first patients in the coming weeks," he said.
Learn more about RevBio at: https://revbio.com/
 Related articles
Related articles
Oral surgery 08 May 2025
Biomaterial sciences are major fields of research in the current era of tissue engineering and regenerative medicine strategies, and these new developments define the new Frontier in many medical...
Implantology 18 November 2024
Implants have been gaining popularity amongst the patients and frequently are being considered as a first treatment option.
Market 07 February 2024
2024 VISION Summit: SprintRay to Unveil Cutting Edge BioMaterial Innovation Lab
SprintRay proudly announces its 2024 VISION Summit, a groundbreaking event highlighting the company’s significant strides in shaping the future of dental 3D printing. This summit will focus on...
Vaccine technology has evolved continuously since its beginning, and mucosal vaccination, including intranasal, sublingual and oral administrations, has recently gained great scientific interest.
News 14 June 2023
The combination creates a diversified dental company with a strong portfolio of leading products across dental waterline treatment, modern dental isolation, and regenerative biomaterials.
 Read more
Read more
Editorials 10 October 2025
With proud smiles and crisp white coats, ninety-three learners from the DDS Class of 2029 and the International Dentist Pathway Class of 2028 marked the start of their dental careers at the UCSF...
Periodontology 10 October 2025
Continuous professional development (CPD) in Periodontology refers to the overall framework of opportunities that facilitate a life-long learning practice, driven by the learner-practitioner and...
TheraBreath, the #1 alcohol-free mouthwash brand in the U.S.*, has introduced a new line of dentist-formulated, clinically tested toothpastes designed to support professional oral care...
News 10 October 2025
New officers and trustees were installed at the Minnesota Dental Association’s Leadership Conference on September 19 in Minneapolis.
News 10 October 2025
Smartee Denti-Technology today announced that Professor Gang Shen, its Chief Scientist and Executive President of TaiKang ByBo Dental, has once again been named to the World’s Top 2% Scientists...