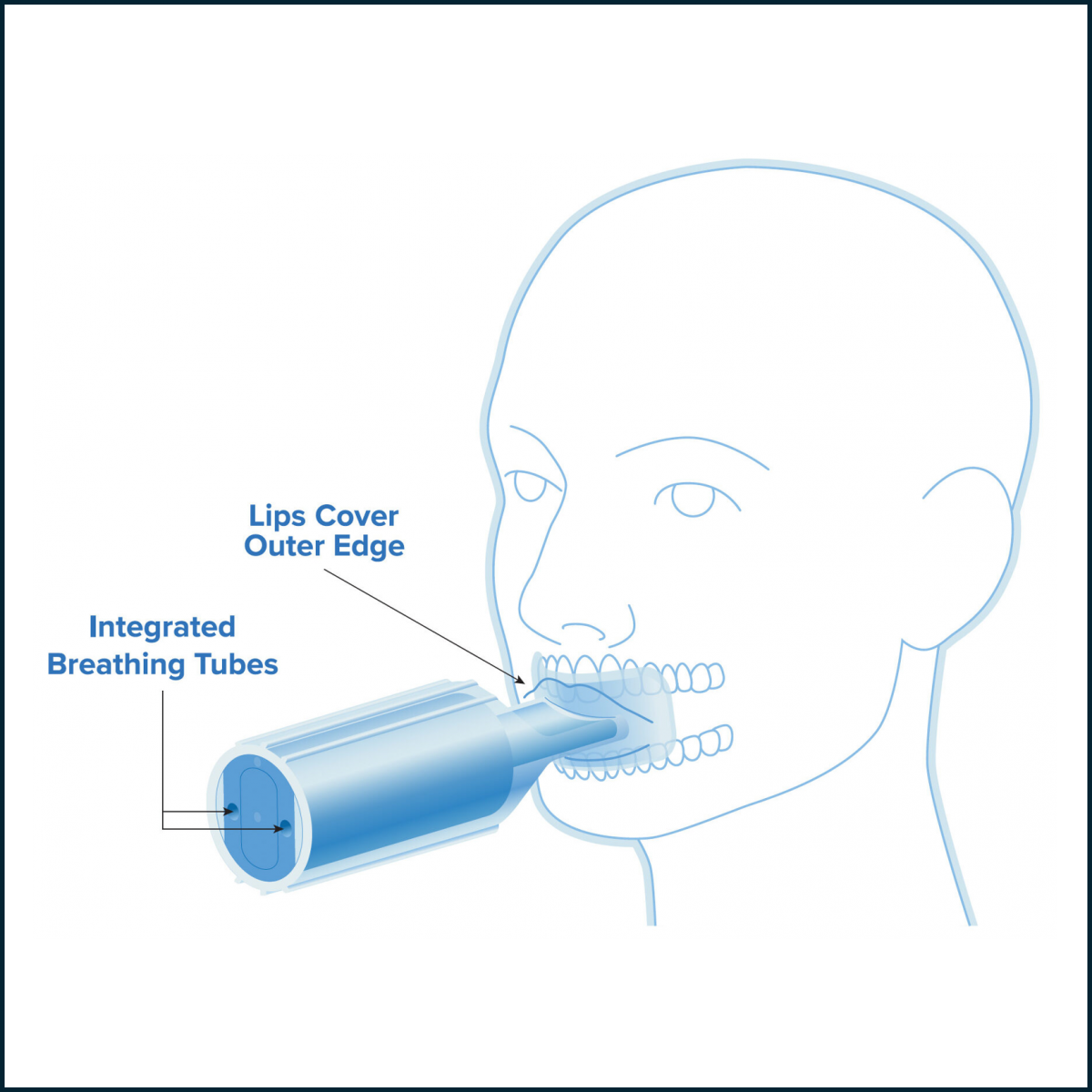
Chemo Mouthpiece Study Shows Significant Pain Reduction
Chemomouthpiece, LLC, a privately held medical device company that develops and commercializes supportive cancer care products, today announced that the peer-reviewed results from its pivotal, multi-center, randomized, controlled trial were published in Supportive Care in Cancer, the official journal of the Multinational Association of Supportive Care in Cancer (MASCC).
The study, “Multi-institutional, randomized, controlled trial to assess the efficacy and tolerability of a reusable, self-contained cryotherapy delivery device,” evaluated the Chemo Mouthpiece, an FDA 510(k) cleared intraoral cryotherapy device, and demonstrated statistically significant reductions in patient-reported oral pain and opioid or analgesic use during chemotherapy treatment.
Use of the Chemo Mouthpiece resulted in a 46% reduction in patient-reported oral pain symptoms compared to the control group. Additionally, there was a 68% reduction in opioid or analgesic use compared to the control group. The device showed efficacy across more than 30 chemotherapy regimens and 17 cancer types. The Chemo Mouthpiece was well-tolerated by patients, with no reported safety issues. Notably, 83% of patients indicated they would recommend the device for patients undergoing chemotherapy.
A Practical Advancement in Oral Mucositis Management
“Cryotherapy is a well-established strategy for mitigating oral mucositis, but its traditional delivery using ice chips presents practical challenges for patients and care teams,” said Stephen Sonis, DMD, DMSc, Senior Lecturer in Oral Medicine at the Harvard School of Dental Medicine and a member of the senior faculty at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital. “The data from this trial indicate that cryotherapy delivered by the Chemo Mouthpiece provided patients with an effective and well-tolerated alternative to ice that could be used throughout their treatment period, both in the clinic and at home. Chemo Mouthpiece-delivered cryotherapy reduced patients’ symptoms and the need to rely on analgesics for pain management.”
Richard Zuniga, MD, Medical Chief of Research at New York Cancer and Blood Specialists and lead study investigator, said, “The data from this rigorous, multi-center trial clearly validate the Chemo Mouthpiece as a safe and effective tool for addressing oral mucositis-related pain and associated opioid use. The scope of chemotherapy regimens and cancer types included in the study underscores the potential to benefit the diverse patient population impacted by oral mucositis. These findings represent a meaningful step forward in supportive oncology care.”
Innovation Driven by Personal Experience
The Chemo Mouthpiece device was developed by company founder and CEO David Yoskowitz, a cancer survivor who experienced firsthand the debilitating effects of oral mucositis from chemotherapy and the lack of effective, tolerable options. At the time, his treatment options included ice chips to mitigate and minimize oral pain; however, they often caused nausea, were difficult to maintain in his mouth, and failed to effectively cool the entire oral cavity. These challenges led him to engineer a better solution.
“This study is not just a scientific milestone; it’s deeply personal,” said Yoskowitz. “It’s incredibly rewarding to see the clinical data confirm the device’s impact on pain reduction and patient quality of life. Not only for me, but for our original goal—to help reduce the suffering associated with oral mucositis for every at-risk patient.”
About the Trial
The randomized trial enrolled 164 patients undergoing chemotherapy and assigned them in a 2:1 ratio to receive either the Chemo Mouthpiece plus best supportive care (arm A) or best supportive care alone (arm B) across 16 U.S. study sites. Inclusion criteria allowed broad tumor diagnoses and chemotherapy regimens. Patients used the device during infusion and for five days post-infusion across two treatment cycles. They completed daily questionnaires to assess oral pain and analgesic use. Device tolerability was also assessed via patient surveys.
Unlike traditional oral cryotherapy approaches, which are generally recommended only for chemotherapy drugs with a short half-life during infusion, the trial included regimens with both short- and long-half-life agents. The portability, tolerability, and user-friendly design of the Chemo Mouthpiece made it practical for patients to continue use at home when chemotherapy agents may still be circulating in the body. This extended use was associated with improved efficacy across a variety of chemotherapy regimens.
The study was published in Supportive Care in Cancer, the official journal of MASCC: Zuniga, R., Dembla, V., Alam, N. et al. Multi-institutional, randomized, controlled trial to assess the efficacy and tolerability of a reusable, self-contained cryotherapy delivery device. Support Care Cancer 33, 732 (2025). https://doi.org/10.1007/s00520-025-09795-x
About Oral Mucositis
Oral mucositis is a painful and often debilitating side effect of chemotherapy, characterized by inflammation and ulceration of the mucous membranes in the mouth. It can interfere with a patient’s ability to eat, drink, speak, and sleep and ultimately can reduce quality of life and delay treatment. Clinical impacts may include:
- Delays or dose reductions in cancer therapy
- Increased risk of infection due to open oral wounds
- Significant weight loss and nutritional challenges
- Greater dependence on opioids and analgesics
- Higher rates of ER visits and hospitalizations
- Increased financial and emotional burden for patients
About the Chemo Mouthpiece
The Chemo Mouthpiece is a self-contained cryotherapy device designed to cool the entire oral cavity during and after chemotherapy infusion. By inducing vasoconstriction in the oral cavity, the device reduces the local presence of chemotherapy drugs, helping to mitigate mucosal injury. Available by prescription only, the device is reusable, pre-filled, and designed for use in both the clinic and at home when chemotherapy may still be circulating, supporting utility across short- and long-half-life regimens. The FDA awarded the Chemo Mouthpiece Breakthrough Device Designation in December 2021, and the device was FDA 510(k) cleared in January 2024.
About Chemomouthpiece, LLC
Chemomouthpiece, LLC is a privately held medical device company headquartered in Wilmington, Delaware. Founded by a cancer survivor, the company’s mission is to bring patient-driven innovation to the forefront of supportive oncology care. The Chemo Mouthpiece® is the company’s flagship product, designed to help reduce the incidence and severity of oral mucositis, one of the most common and painful side effects of chemotherapy, impacting up to 80% of patients depending on cancer type.
This release contains forward-looking statements regarding Chemomouthpiece, LLC, including its potential benefits and future development plans, which are subject to risks and uncertainties that may cause actual results to differ. The company undertakes no obligation to update these statements except as required by law.
Source: www.chemomouthpiece.com
 Related articles
Related articles
Implantology 20 November 2019
Osteointegration of titanium implant in patients treated with antiangiogenic targeted chemotherapy
Recent advances in dental implants have led to an increase in the number of patients treated, with survival rates of more than 95% in a healthy population. ...
Products 16 November 2023
Ascentcare Dental Products, a dental product development company, announces the recent issuance of US Patent 11,737,739 by the United States Patent and Trademark Office. Granted August 29, 2023, this...
Endodontics 23 December 2025
Serious consequences of endodontics phlegmons and abscesses of the neck: a retrospective study
In the last years there has been an alarming increase in number of patients admitted to the emergency room (E.R.) with the diagnosis of neck abscesses and phlegmons of odontogenic origin requiring...
HSDM researchers uncover a key mechanism for bone health that could lead to potential therapeutic agents to mimic the effects of exercise
News 18 December 2025
Dental health isn’t just about teeth—it’s about overall well-being, learning, and family life.
 Read more
Read more
Implantology 24 December 2025
Soft tissue management in aesthetic implantology: clinical case with 15-year follow-up
Implant therapy is widely recognized as an effective and predictable solution for replacing missing teeth, supported by extensive scientific evidence.
Editorials 24 December 2025
News 24 December 2025
Medit, a global leader in 3D dental scanning solutions, announced the launch of “Medit’s 2025 End of Year Promotion,” a limited-time campaign delivering significant benefits to dental...
News 24 December 2025
Recognized globally for her authority in CAD/CAM and CBCT, Dr. Gupta brings nearly 2 decades of expertise to guide CDOCS faculty and members forward.