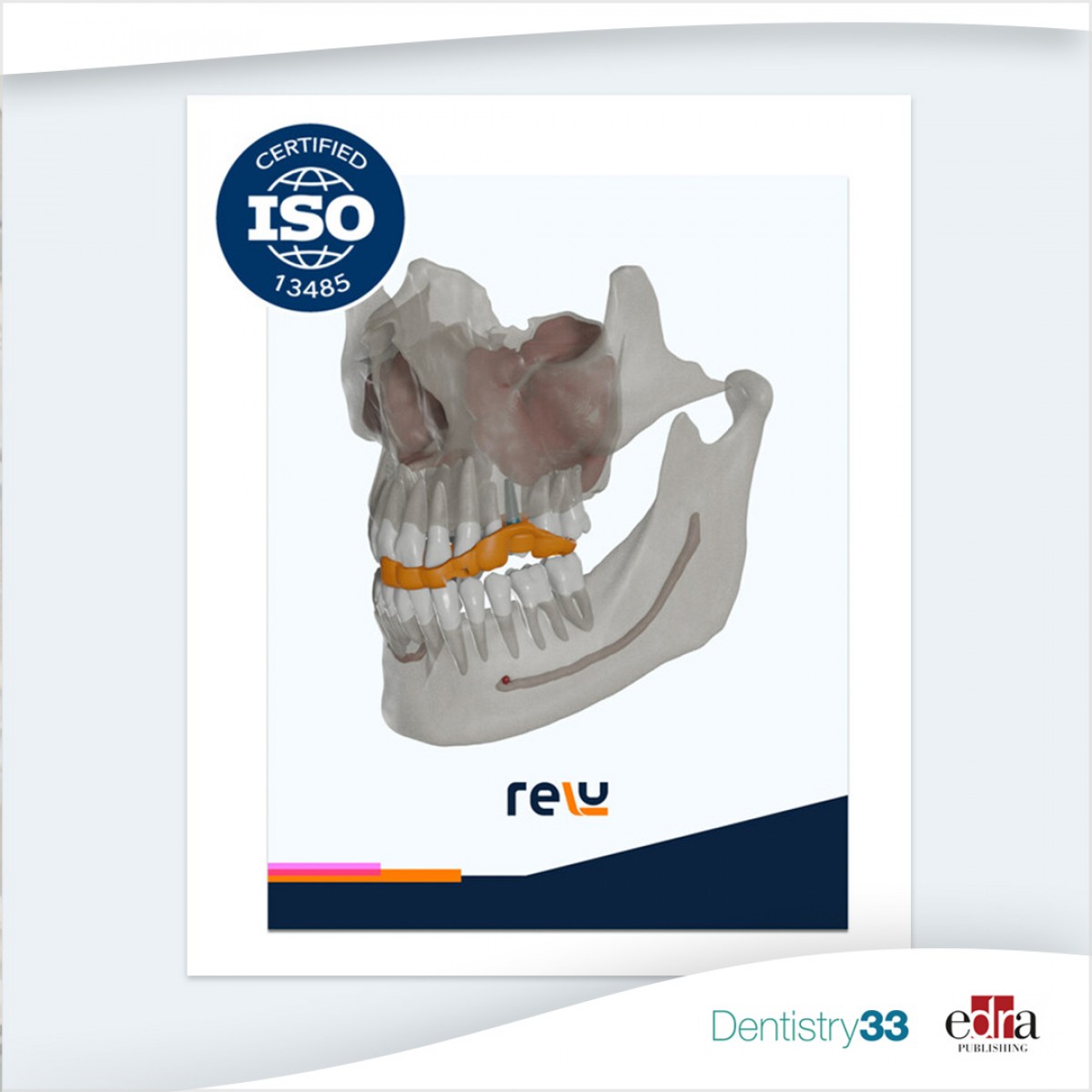
Relu obtains global quality standard for medical device companies
Relu, an OEM software provider in the dental industry, obtained ISO13485 certification from EU Notified Body Kiwa Dare. ISO13485 is the global quality standard for medical device companies, according to a Sept. 12 news release.
The company provides enterprise-grade artificial intelligence (AI) for workflow automation in dental treatment software. They partner closely with leading dental software companies to make their software more valuable and user-friendly with AI.
This new certification will help to increase the quality of Relu's product, and it will also reduce the regulatory work of partners when integrating AI in their medical device software. Relu took this step to bring better AI innovation to both the dentist and the patient.
"This certification demonstrates our commitment to international quality and regulatory standards," said Holger Willems, co-founder & CEO at Relu in the news release. He added that the company wants to build AI that people can trust.
"We've achieved ISO13485, underscoring our commitment to quality and safety," said Bindu Saran, director at Relu and former global head of orthodontic technology at Straumann Group. "This certification reassures our global partners of our dedication to innovative AI solutions for implantology and orthodontics. It's a clear mark of trust for all involved."
About Relu
Founded in 2019, Relu has grown to a 15-person team with a vision to make dental treatments safer, faster and better with AI. They partner with the biggest companies in dentistry to embed Relu's AI plug-in into the leading dental treatment software. The company recently landed investments from industry veterans like Dental Innovation Alliance. Relu is based in Leuven, Belgium. Learn more at www.relu.eu.
About ISO13485
The ISO 13485:2016 is an international standard required to sell medical devices in many countries. It specifies the requirements for a medical quality management system. Learn more about the standard at www.iso.org/standard/59752.html.
 Related articles
Related articles
Over one million small and medium-sized (SMB) dental and healthcare practices play a vital role in delivering patient care across the U.S.
Products 23 September 2025
Dentech’s Practice Management Software Has Robust Feature Set
Dentech’s state-of-the-art practice management software fits right into the day-to-day operations of a dental practice of any size.
Products 03 September 2025
The latest version of Planmeca’s all-in-one dental software, Planmeca Romexis 7, is now available for purchase
The software’s AI works like a teammate, not just a tool, while tackling staffing shortages, insurances issues, payment obstacles, and more.
Products 26 November 2024
DentalMonitoring Enhances Orthodontic Software With FDA-Cleared AI Technology
DentalMonitoring, the leader in artificial intelligence (AI)-powered remote monitoring for orthodontics, has released a major software update introducing features and indications based on the...
 Read more
Read more
Much like EMTs rushing to the scene after an accident, stem cells hurry to the site of a skull fracture to start mending the damage. A new finding has uncovered the signaling mechanism that triggers...
Products 05 November 2025
SimplyTest has launched a groundbreaking saliva-based test to detect high-risk strains of oral human papillomavirus (HPV), a major cause of oropharyngeal cancers.
News 05 November 2025
Perimetrics, Inc., a dental technology company pioneering quantitative diagnostics, announced today that the U.S. Food and Drug Administration (FDA) has granted clearance for the InnerView...
News 05 November 2025
On October 15, open enrollment for Medicare began nationwide. Hundreds of thousands of seniors in New Jersey will once again face the challenge of finding the right Medicare coverage, including the...
Digital Dentistry 04 November 2025
Digitalisation is an expanding field in dentistry and implementation of digital teaching methods in dental education is an essential part of modern education.