FDA Clears Wesper Device For At-Home Sleep Apnea Diagnosis
Now from the comfort of your home, using Wesper can lead to real diagnoses for common sleep issues.
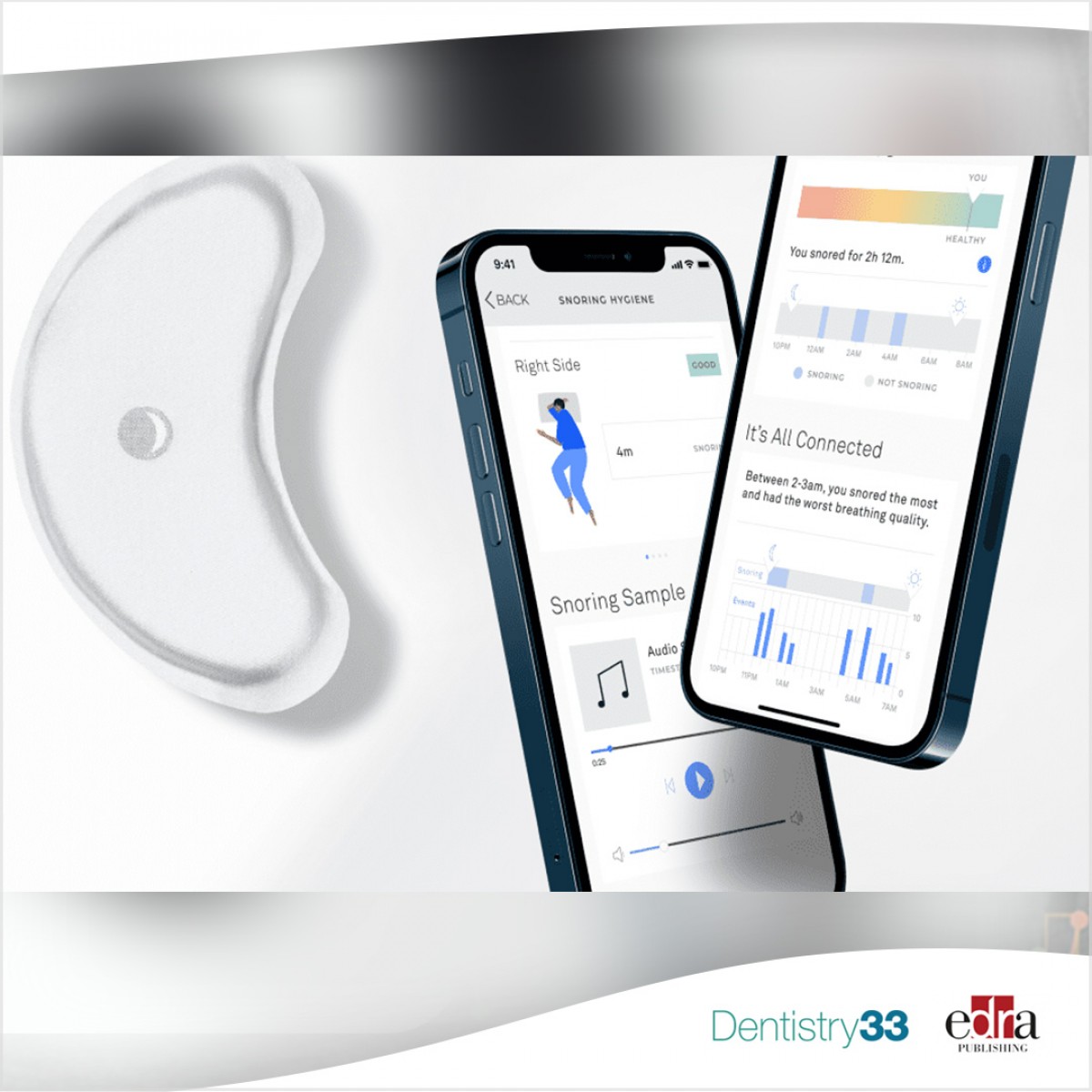
Wesper - the first of its kind sleep health platform officially launched their Wesper Lab, the successor device to their successful Wesper Lite. The Wesper has been cleared by the U.S. Food and Drug Administration and has received its 510(K). Wesper’s sleep health platform assists trained personnel in the diagnosis of sleep apnea with data from a body-worn component that the user can apply in the comfort of their own bed.
Wesper has one of the highest degrees of correlation with PSGs compared to any other Type 3 device. The platform monitors several key parameters that you can’t find anywhere else, except sleep labs. In addition to sleep time, sleep efficiency, sleep latency, and heart rate, Wesper also reports on body positions throughout the night, airflow, respiratory effort, and snoring. Wesper supports and even encourages, multiple nights of testing at no additional cost. Evidence is growing that a single-night test does not account for multi-night variability and Wesper believes multi-night testing will become the standard of care, even for lab-based studies.
Wesper is cloud based: the data is available for interpretation in near real-time, reducing delays and errors associated with equipment that must be returned to the clinic before an interpretation can take place. This is a major pain point for many of their customers. Wesper will connect users virtually to a sleep specialist – putting them on the track to personalized treatment options and better sleep, once and for all. Wesper has distilled the power of a sleep lab into an easy-to-use patch, allowing anyone to be diagnosed and get treated for their sleep issues with the guidance of a sleep specialist.
Wesper is focused on transforming the lives of anyone with chronic sleep problems and those with suspicions about living with a sleep disorder, such as sleep apnea. Sleep apnea is one of the most common sleep disorders in the United States, with approximately 54M adults suffering from the condition.
Untreated sleep apnea increases the risk of dying from cardiovascular disease 5-fold, as well as elevating the risk for cancer and Alzheimer's disease. Wesper’s sleep-lab-in-a-patch solution was developed in close collaboration with practicing physicians researchers and the nation's leading sleep experts and brings the highest levels of accuracy to people’s homes.
“Improving your sleep used to be a very lonely and complicated process and we are here to change that,” says Dr. Amir Reuveny, CEO and co-founder of Wesper “We are committed to our users every step of the way to getting high-quality sleep.”
About Wesper
For individuals who strive to live healthier lives, Wesper is at the forefront of health technology - providing users with the potential for better sleep through an innovative platform that integrates optimum comfort and virtual guidance. Wesper’s commitment to comfort, accessibility, and technological advancement enables it to focus on building healthcare tools tailored to customers’ unique needs. Wesper has redefined sleep health by creating a seamless path to personalized solutions based on granular, clinical-grade data. Established in 2017 and headquartered in NY, as a spinout of Cornell Tech University, Wesper has recently received funding from leading VCs and employs approximately 20 distinguished professionals. With media outlets such as Forbes, TED & TechCrunch taking notice, Wesper is establishing a strong foundation for itself and is looking forward to improving the lives of billions around the globe.
Source: https://wesper.co/
 Related articles
Related articles
Products 26 November 2024
DentalMonitoring Enhances Orthodontic Software With FDA-Cleared AI Technology
DentalMonitoring, the leader in artificial intelligence (AI)-powered remote monitoring for orthodontics, has released a major software update introducing features and indications based on the...
News 15 October 2024
Relu Secures FDA Clearance and CE Mark Approval for Revolutionary Dental AI Tool
Relu, a pioneer in artificial intelligence (AI)-assisted segmentation for dental labs and software companies, proudly announces the dual achievement of 510(k) clearance by the U.S. Food and Drug...
Market 11 April 2024
Orthobond Revolutionizes Medical Device Safety With FDA Approval of Ostaguard Coating
Orthobond Corporation, a leader in covalently-bound antibacterial surface technologies with broad applications in the medical device industry, announced that the U.S. Food and Drug Administration...
AIOMEGA, a Texas biomedical company, announced that AIO BREATHE, their medical device that treats Obstructive Sleep Apnea, has been cleared by the Food and Drug Administration.
The U.S. Food and Drug Administration (FDA) has approved Dr. Jonathan An from the UW Department of Oral Health Sciences to lead the first-ever study to evaluate rapamycin in older adults with...
 Read more
Read more
Oral pathology 24 October 2025
Isolation and characterization of dental pulp stem cells from a supernumerary tooth
Dental pulp stem cells (DPSCs) were primarily derived from the pulp tissues of primary incisors and permanent third molar teeth, whereas no report to our knowledge has yet been documented on deriving...
Editorials 24 October 2025
From mentoring workshops to leadership insights, the last week’s IU School of Dentistry (IUSD) fall faculty conference and staff retreat brought faculty and staff together respectively for two days...
Products 24 October 2025
At the American Academy of Periodontology’s Annual Meeting, Carestream Dental continues to deliver what’s next in dentistry with the launch of CS 3D.
News 24 October 2025
As dental professionals prepare to wrap up 2025, many are setting ambitious goals for the year ahead, yet few have a clear, actionable plan to achieve them.
News 24 October 2025
The Yankee Dental Congress will take place from January 29, 2026, through January 31, 2026, at the Thomas M. Menino Convention & Exhibition Center in Boston.