Denti.AI Earns FDA Clearance for Breakthrough Dental AI Solutions
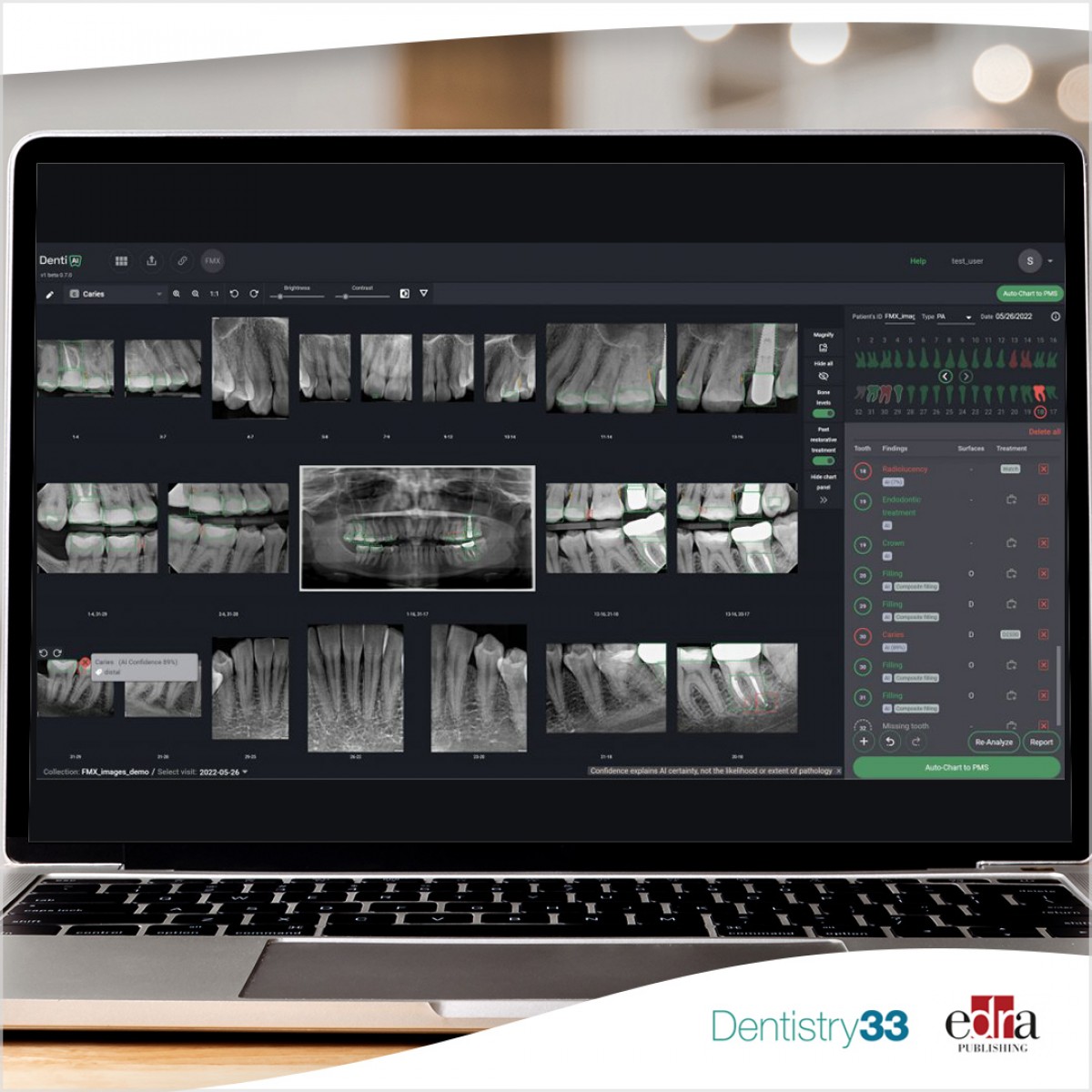
Denti.AI, a pioneer in leveraging Artificial Intelligence for dental pathology detection, odontogram, and periodontal charting automation, proudly announces the 510(k) clearance by the FDA of its innovative product, Denti.AI Detect. This state-of-the-art imaging solution is engineered to aid dental professionals in identifying prevalent dental diseases such as caries, periapical lesions, and bone loss from dental x-rays. Clinical studies reveal a notable enhancement in disease detection by all the two dozen participating dentists, unlocking 26% extra treatment opportunities with Denti.AI Detect.
Following the prior clearance of Denti.AI Auto-Chart in November 2022, a breakthrough in automating odontogram charting of restorations, root canals, implants, crowns, and missing teeth, Denti.AI Detect now integrates with Auto-Chart to further refine disease charting and treatment plan coding in multiple integrated Practice Management Systems (PMS). This integration represents a significant stride towards optimizing dental practice workflows and enhancing patient care.
A key distinguishing feature of Denti.AI Detect is its industry-first FDA clearance for disease detection on panoramic images. Denti.AI also stands as the only dental AI company to have secured clearance for all three major dental concerns: caries, periapical radiolucencies, and bone levels, marking a monumental achievement in the dental technology landscape.
Dr. Edward Gelfand, owner of over 30 dental practices and partner of the largest Canadian DSO, extolled the effectiveness of Denti.AI Detect saying, “The ability of Denti.AI Detect to uncover missed diseases has been a game-changer in not only identifying treatment opportunities but also in educating our patients. Our best dentists attest to the indispensable nature of this tool in their practice.”
Before its FDA clearance, Denti.AI Detect had already been licensed by Canada Health and extensively employed across multiple dental practices in Canada, further demonstrating its practical utility and reliability.
Dr. Jonathan B. Levine, owner of the prestigious multi-specialty clinic JBL NYC and chairman/founder of innovative dental technology company GLOScience, shared his positive experience, “After the rigorous research of Denti.AI Detect in our clinic over many months, I am thrilled to finally become a customer. The technology has proven itself invaluable.”
Denti.AI founder and CEO, Dmitry Tuzoff, reflected on the journey, “The realization of Denti.AI Detect clearance signifies countless hours of dedication from our team. The introduction of panoramic imaging technology, especially, propels our product to the forefront, addressing the growing usage of such imaging practices across North America.”
As part of its launch celebration, Denti.AI is offering special pricing terms for Denti.AI Detect & Auto-Chart until the end of the year. Interested parties are encouraged to explore this opportunity by filling out the form at www.denti.ai.
About Denti.AI
Established in 2017, Denti.AI employs AI to decipher pathologies, past treatments, and anatomical features from dental x-rays and voice commands, facilitating early disease detection, enhancing productivity for dentists and hygienists, and ensuring accurate record-keeping. Integrated with practice management systems, Denti.AI’s imaging and voice AI products are automating workflows for dental groups across North America, boasting hundreds of active users.
Source: visit www.denti.ai
 Related articles
Related articles
Products 22 December 2025
AI dental company announces 510(k) clearance from the U.S. Food and Drug Administration for Overjet CBCT Assist.
News 05 November 2025
Perimetrics, Inc., a dental technology company pioneering quantitative diagnostics, announced today that the U.S. Food and Drug Administration (FDA) has granted clearance for the InnerView...
Products 26 November 2024
DentalMonitoring Enhances Orthodontic Software With FDA-Cleared AI Technology
DentalMonitoring, the leader in artificial intelligence (AI)-powered remote monitoring for orthodontics, has released a major software update introducing features and indications based on the...
News 15 October 2024
Relu Secures FDA Clearance and CE Mark Approval for Revolutionary Dental AI Tool
Relu, a pioneer in artificial intelligence (AI)-assisted segmentation for dental labs and software companies, proudly announces the dual achievement of 510(k) clearance by the U.S. Food and Drug...
Market 11 April 2024
Orthobond Revolutionizes Medical Device Safety With FDA Approval of Ostaguard Coating
Orthobond Corporation, a leader in covalently-bound antibacterial surface technologies with broad applications in the medical device industry, announced that the U.S. Food and Drug Administration...
 Read more
Read more
Endodontics 13 January 2026
Regenerative endodontic treatment has provided a treatment option that aims to allow root maturation.
Editorials 13 January 2026
Pitt’s New Digital Dentistry Labs to Provide State-of-the-Art Education and Patient Care
Three new dentistry labs at the University of Pittsburgh School of Dental Medicine will give students and residents hands-on experience with cutting edge digital dental technology while providing...
Products 13 January 2026
Revolutionizing Pediatric Dentistry with Dr. Josh Solomon: SDI Stela & Bioclear Insights
Join pediatric dentist Dr. Josh Solomon as he discusses the cutting-edge SDI Stela self-curing composite system and the Bioclear matrix system, and how these products are transforming Class II...
News 13 January 2026
Curve Dental, the leading cloud-native, all-in-one dental practice management platform, today announced its recognition as a market leader in The 2026 Dental Technology Landscape: Cloud, AI, and the...
News 13 January 2026
Patterson Companies Inc. has announced the appointment of Patrik Eriksson as Patterson Dental’s North American president. Eriksson brings a wealth of experience in the dental and medical technology...